Calculate the Kc Value for the a -protein Binding Reaction.
Calculations where K is already known working backwards 4. If Q Kc the reaction will proceed to the right.

Physical Chemistry When Are Kp And Kc Values Equal Chemistry Stack Exchange
Calculate Kp at.

. Solution for Calculate Kc for the reaction below at 727C given that the equilibrium concentration of NO is 129 M Br2 is 1052 M and NOBr is 0423M2NOBrg. K for the reaction below has a value of 55 at a certain given temperature. Calculate the value of the equilibrium constant for the reaction.
Calculate the molar concentration of all three substances present at equilibrium and hence the value of Kc. So that the stoichiometry of the reaction alone can be used to find the concentrations of FeNCS 2. 19 moles of HI are added to an evacuated sealed 10-L container and allowed to decompose according to the chemical equation.
H 2 Ba s X kJmol. What is the energy of the molecules of the products. This experiment is to calculate the formation constant which leaves us in a bit of a quandary.
Calculate the value of the equilibrium constant for the reaction 4NH3g3O2g2N2g6H2Og given the following equilibrium constants at a certain temperature. N2O4g2NO2g Express your answer using two significant figures. Up to 24 cash back contain 0220 moles of ester.
Calculate the number of moles of HBr present in an. Otherwise you will need to take a more complicated. Remember a differential rate law expresses a reaction rate as a function of reactant concentrations.
A A 100-L flask containing 00500 mol of NOg 00155 mol of. Use this link look up the Δ H f values 2 NaH g BaCl 2s - H 2 Ba s 2 NaCl s Δ H -536 kJmol. This is the reverse of the last reaction.
Enter the reactants products coefficients and concentrations in the input field. Because a large excess of Fe3 is used it is reasonable to assume that all of the SCN- is converted to FeSCN2. Calculate k at 398 K.
Now click the button Calculate Equilibrium Constant to get the output. What is the numerical value of Kc for the following reaction if the equilibrium mixture contains 032 M CO2 0036 M H2 023 M CO and 028 M H2O. The only thing that changes an equilibrium constant is a change of temperature.
The equilibrium in the hydrolysis of esters. At 1 atm and 25 C NO 2 with an initial concentration of 100 M is 33 10-3 decomposed into NO and O 2. Finally the equilibrium constant for the given chemical reaction will be displayed in the.
The K c expression is. Check Your Learning Calculate the reaction quotient and determine the direction in which each of the following reactions will proceed to reach equilibrium. The importance is.
Kc 126 103. At 473 K this reaction has a Kc value of 00133. What is Q-value of nuclear reaction Class 12.
H2 g I2 g 2HI g. At room temperature this value is approximately 4 for this reaction. 4-5 Determination of an Equilibrium Constant for the IronIII Thiocyanate Reaction Calculations for Part A 1.
All reactant and product concentrations are constant at equilibrium. Equilibrium is when the rate of the forward reaction equals the rate of the reverse reaction. For the reaction Xg2Yg3Zg Kp 171102 at a temperature of 225 C.
G 0 and Qc Kc or Kp at the start of the reaction. G Gibbs Free Energy K Equilibrium Constant and Q Reaction Quotient are related as follows. The procedure to use the equilibrium constant calculator is as follows.
If Q Kc the reaction will proceed to the left. Use the Δ H and balanced chemical equation below and calculate the Δ H f of H 2 Ba s. From the question N₂O₄ 0035 M and NO₂ 019 M.
VIDEO Calculate Δ H DELTA H Demonstrated Example 2. The numerical value of the equilibrium constant Kc for the given reaction is 10314 M. BrO 3- 5Br- 6H 3Br 2 3H 2 O.
The specific rate constant k for a reaction is 044 s-1 at 298 K and the activation energy is 245kJmol. N₂O₄g 2NO₂g The equilibrium constant Kc for the given reaction is given by. If the reaction is carried out under standard conditions unit concentrations and pressures and at a temperature that corresponds to a table of thermodynamic values usually 29815 K then you can subtract the standard Gibbs Free Energy of Formation DeltaG_f of the reactants from those of the products.
Given a reaction the equilibrium constant also called or is defined as follows. The reaction will proceed to form products. The given reaction is.
Consider the equilibrium reaction 1. The equilibrium constant K for binding of hemoglobin for carbon monoxide CO is about 200-300 times greater than for O2. Be sure to take into account the dilution that occurs when the.
For example consider the reaction. The reaction will proceed in the forward direction. What is the numerical value of Kc for the following reaction if the equilibrium mixture contains 0026 M N2O4 and 024 M NO2.
Equilibrium constants arent changed if you change the concentrations of things present in the equilibrium. A reversible reaction can proceed in both the forward and backward directions. Consider the following reaction where Kc 29010-2 at 1150 K.
Calculate and record in lab notebook the FeSCN2 in each solution and its absorbance. 271 1010 s-1 Given the following data for the NH4 NO2- N2 2 H2O reaction. The Q-value for a reaction in nuclear physics and chemistry is the amount of energy consumed or emitted during the nuclear reaction.
G 0 and Qc Kc or Kp at equilibrium and no more changes in the concentration of the mixture. Calculate the value of Kc. The position of equilibrium is changed if you change the concentration of something present in the mixture.
Calculate the value of the equilibrium constant for the decomposition of PCl 5 to PCl 3 and Cl 2 at this temperature. Subsequent measurement of the. 2 SO3 g 2 SO2 g O2 g A reaction mixture was found to contain 47110-2 moles of SO3 - 16777612.
Kc 10314 M Hence the numerical value of the equilibrium constant Kc for the given reaction is 10314 M. As long as you keep the temperature the same whatever proportions of acid and alcohol you mix together once equilibrium is reached K c always has the same value. Value ranges from 0 to 10 with 10 indicating a perfect fit.
N2g3H2g2NH3g for which Δn2132. According to Le Chateliers Principle the. Using the data listed below in Table 12-5 calculate the order for each reactant the overall reaction order and the value of the rate constant for the following reaction.
-Use the initial concentrations given to calculate the value of Kc. If Q Kc the reaction is at equilibrium.

At 473 K Equilibrium Constant Kc For Decomposition Of Phosphorus Pentachloride Pcl5 Is 8 3 10 3 If Decomposition Is Depicted As Pcl5 G Pcl3 G Cl2 G Drh 124 0kjmol 1

At 473 K Equilibrium Constant Kc For Decomposition Of Phosphorus Pentachloride Pcl5 Is 8 3 10 3 If Decomposition Is Depicted As Pcl5 G Pcl3 G Cl2 G Drh 124 0kjmol 1
What Is The Value Of R In Kp Kc Rt N Quora
15 4 Calculating The Equilibrium Constant From Measured Equilibrium Concentrations Chemistry Libretexts
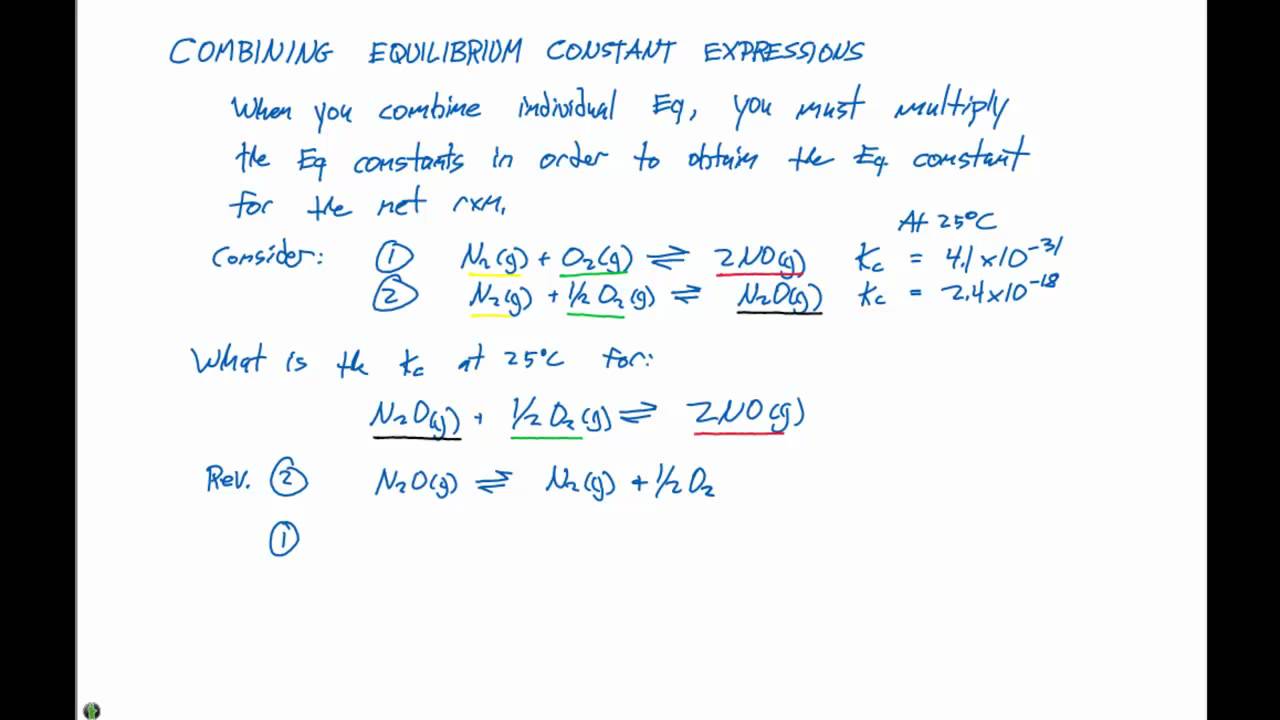
15 3 Combining Equilibrium Constants Youtube

Properties Of The Equilibrium Constant Video Khan Academy

Writing Equilibrium Constant And Reaction Quotient Expressions Video Khan Academy
What Is Qc In Chemical Equilibrium And What Is The Relation Between Qc And Kc Quora
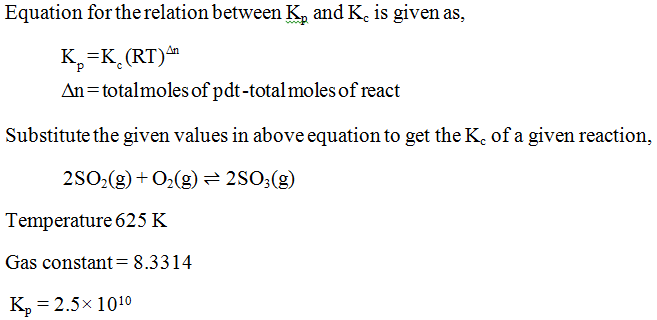
Answered Calculate Kc For The Following Bartleby
What Is Qc In Chemical Equilibrium And What Is The Relation Between Qc And Kc Quora
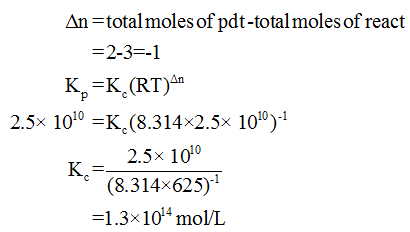
Answered Calculate Kc For The Following Bartleby
What Is The Value Of R In Kp Kc Rt N Quora

In A Fission Reaction 92 236 U Rarr 117 X 117y N N The Binding Energy Per Nucleon Of X And Y Is 8 5 Mev Whereas Of 236 U Is
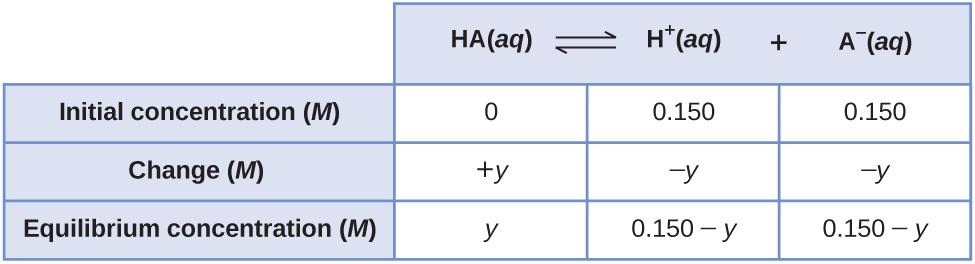
13 4 Equilibrium Calculations Chemistry
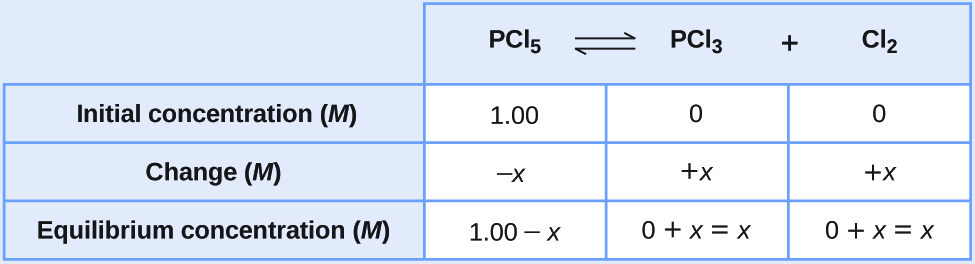
13 4 Equilibrium Calculations Chemistry
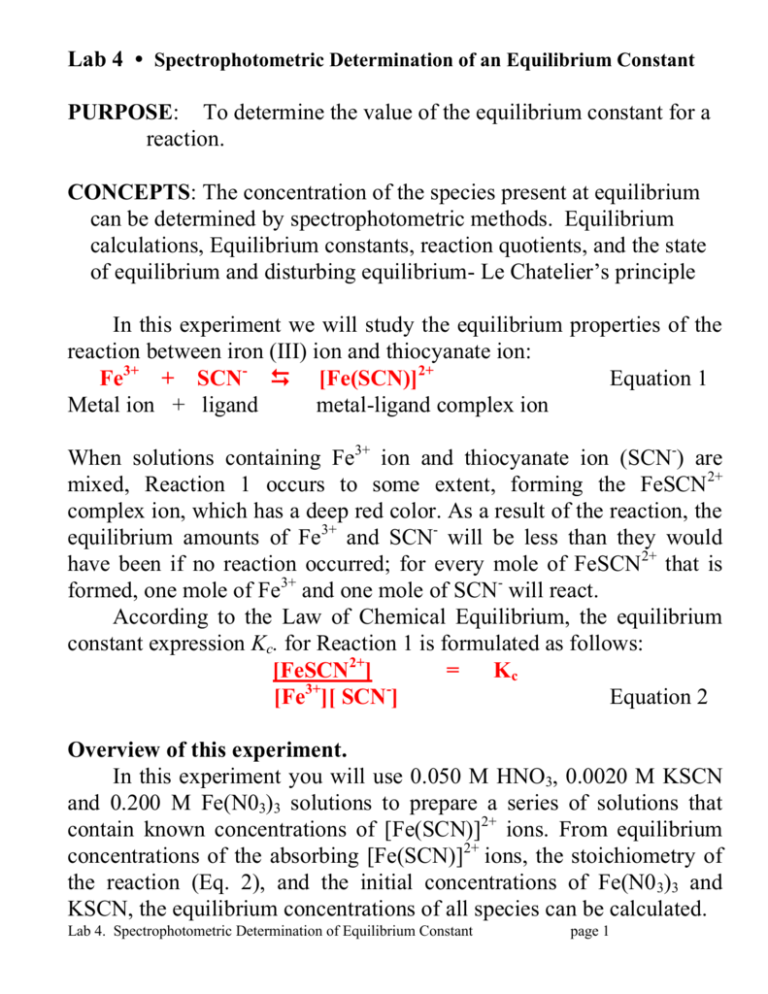
Purpose To Determine The Value Of The Equilibrium Constant For A

Ice Table Practice Problems Initial Concentration Equilibrium Concentration Kc Part 1 Youtube


Comments
Post a Comment